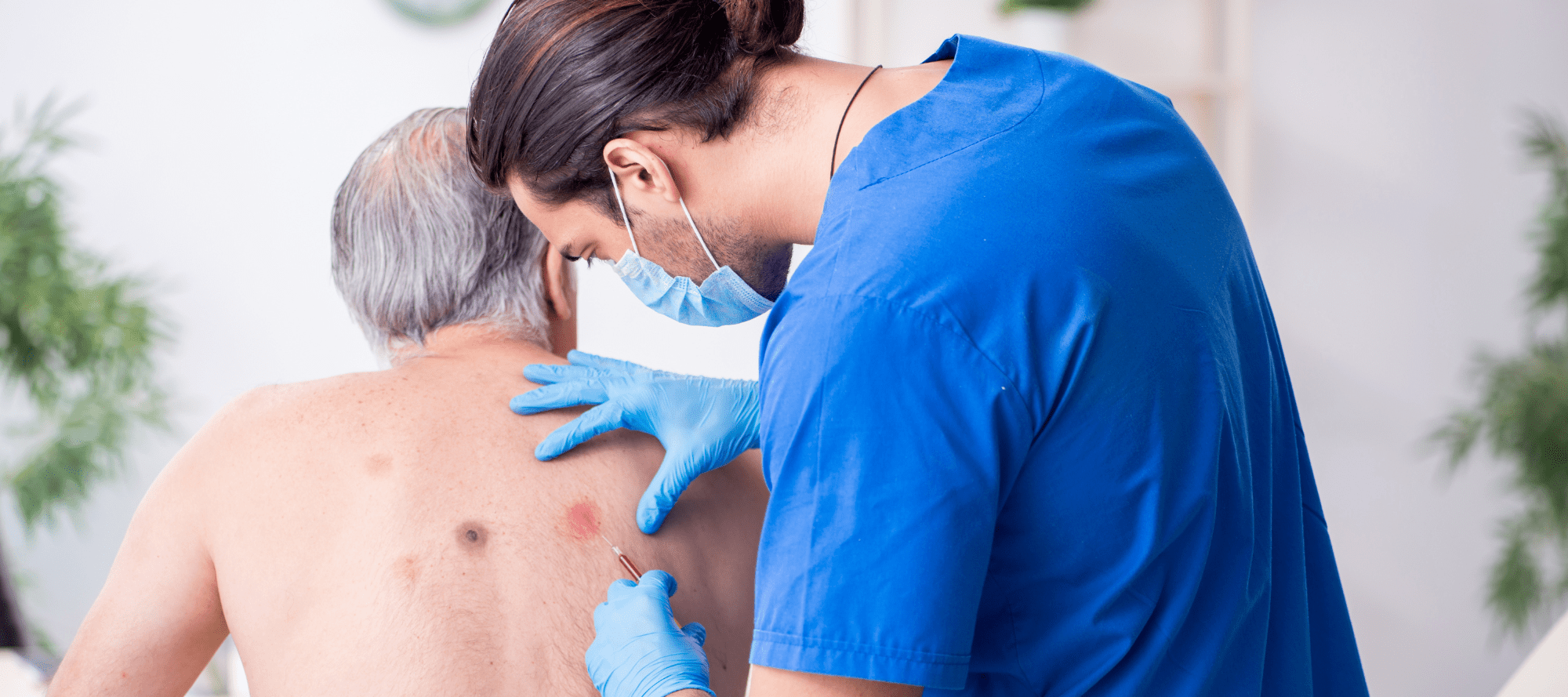
- Case Report of Leprosy in Central Florida, USA, 2022
A 54-year-old man sought treatment at a dermatology clinic for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. He denied any domestic or foreign travel, exposure to armadillos, prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. He has resided in central Florida his entire life, works in landscaping, and spends long periods of time outdoors. Biopsies of multiple sites demonstrated a diffuse dermal infiltrate composed of disorganized aggregates of foamy histiocytes and lymphocytes. Fite stains revealed acid-fast bacilli within histiocytes and cutaneous nerve twigs, a pathognomonic finding of leprosy. He was referred to an infectious disease specialist who, under the direction of the National Hansen’s Disease Program, prescribed triple therapy with dapsone, rifampin, and clofazimine. Florida, USA, has witnessed an increased incidence of leprosy cases lacking traditional risk factors. Those trends, in addition to decreasing diagnoses in foreign-born persons, contribute to rising evidence that leprosy has become endemic in the southeastern United States. Travel to Florida should be considered when conducting leprosy contact tracing in any state. - A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection
Studies have demonstrated that at least 20% of individuals infected with SARS-CoV-2 remain asymptomatic. Although most global efforts have focused on severe illness in COVID-19, examining asymptomatic infection provides a unique opportunity to consider early immunological features that promote rapid viral clearance. Here, postulating that variation in the human leukocyte antigen (HLA) loci may underly processes mediating asymptomatic infection, researchers enrolled 29,947 individuals, for whom high-resolution HLA genotyping data were available, in a smartphone-based study designed to track COVID-19 symptoms and outcomes. This discovery cohort (n = 1,428) comprised unvaccinated individuals who reported a positive test result for SARS-CoV-2. Researchers tested for association of five HLA loci with disease course and identified a strong association between HLA-B*15:01 and asymptomatic infection, observed in two independent cohorts. Suggesting that this genetic association is due to pre-existing T cell immunity, we show that T cells from pre-pandemic samples from individuals carrying HLA-B*15:01 were reactive to the immunodominant SARS-CoV-2 S-derived peptide NQKLIANQF. The majority of the reactive T cells displayed a memory phenotype, were highly polyfunctional and were cross-reactive to a peptide derived from seasonal coronaviruses. The crystal structure of HLA-B*15:01–peptide complexes demonstrates that the peptides NQKLIANQF and NQKLIANAF (from OC43-CoV and HKU1-CoV) share a similar ability to be stabilized and presented by HLA-B*15:01. Finally, we show that the structural similarity of the peptides underpins T cell cross-reactivity of high-affinity public T cell receptors, providing the molecular basis for HLA-B*15:01-mediated pre-existing immunity. - Delayed intubation associated with in-hospital mortality in patients with COVID-19 respiratory failure who fail heated and humified high flow nasal canula
Advanced respiratory support modalities such as non-invasive positive pressure ventilation (NiPPV) and heated and humidified high flow nasal canula (HFNC) served as useful alternatives to invasive mechanical ventilatory support for acute respiratory failure (ARF) during the peak of the SARS-CoV-2/COVID-19 pandemic. Unlike NiPPV, HFNC is a newer modality and its role in the treatment of patients with severe ARF is not yet clearly defined. Furthermore, the characteristics of responders versus non-responders to HFNC have not been determined. Although recent evidence indicates that many patients with ARF treated with HFNC survive without needing intubation, those who fail and are subsequently intubated have worse outcomes. Given that prolonged use of HFNC in patients with ARF might exacerbate patient self-inflicted lung injury, researchers hypothesized that among those patients with ARF due to COVID-19 pneumonia, prolonged HFNC beyond 24 h before intubation would be associated with increased in-hospital mortality. This was a retrospective, multicenter, observational cohort study of 2720 patients treated for ARF secondary to SARS-CoV-2/COVID-19 pneumonia and initially managed with HFNC within the Banner Health system during the period from March 1st, 2020, to July 31st, 2021. In the subgroup of patients for went from HFNC to IMV, we assessed the effect of the duration of HFNC prior to intubation on mortality. 1392 (51%) were successfully treated with HFNC alone and 1328 (49%) failed HFNC and were intubated (HFNC to IMV). When adjusted for the covariates, HFNC duration less than 24 h prior to intubation was significantly associated with reduced mortality. Among patients with ARF due to COVID-19 pneumonia who fail HFNC, delay of intubation beyond 24 h is associated with increased mortality - Blood Group A Enhances SARS-CoV-2 Infection
Among risk factors for SARS-CoV-2, ABO(H) blood group antigens have been one of the most recognized predictors of infection. However, the mechanisms whereby ABO(H) antigens influence susceptibility to COVID-19 remain incompletely understood. The receptor binding domain (RBD) of SARS-CoV-2, which facilitates host cell engagement, bears significant similarity to galectins, an ancient family of carbohydrate binding proteins. As ABO(H) blood group antigens are carbohydrates, we compared the glycan binding specificity of the SARS-COV-2 RBD with galectins. Similar to the binding profile of several galectins, the RBDs of SARS-CoV-2, including Delta and Omicron variants, exhibited specificity for blood group A. Not only did each RBD recognize blood group A in a glycan array format, but each SARS-CoV-2 virus likewise displayed a preferential ability to infect blood group A expressing cells. Preincubation of blood group A cells with a blood group binding galectin specifically inhibited the blood group A enhancement of SARS-CoV-2 infection, while similar incubation with a galectin that does not recognize blood group antigens failed to impact SARS-CoV-2 infection. These results demonstrate that SARS-CoV-2 can engage blood group A, providing a direct link between ABO(H) blood group expression and SARS-CoV-2 infection. - Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort
Lack of specific definitions of clinical characteristics, disease severity, and risk and preventive factors of post-COVID-19 syndrome (PCS) severely impacts research and discovery of new preventive and therapeutics drugs. This prospective multicenter cohort study was conducted from February 2020 to June 2022 in 5 countries, enrolling SARS-CoV-2 out- and in-patients followed at 3-, 6-, and 12-month from diagnosis, with assessment of clinical and biochemical features, antibody (Ab) response, Variant of Concern (VoC), and physical and mental quality of life (QoL). Outcome of interest was identification of risk and protective factors of PCS by clinical phenotype, setting, severity of disease, treatment, and vaccination status. Researchers used SF-36 questionnaire to assess evolution in QoL index during follow-up and unsupervised machine learning algorithms (principal component analysis, PCA) to explore symptom clusters. Severity of PCS was defined by clinical phenotype and QoL. Researchers also used generalized linear models to analyse the impact of PCS on QoL and associated risk and preventive factors. Among 1796 patients enrolled, 1030 (57%) suffered from at least one symptom at 12-month. PCA identified 4 clinical phenotypes: chronic fatigue-like syndrome (CFs: fatigue, headache and memory loss, 757 patients, 42%), respiratory syndrome (REs: cough and dyspnoea, 502, 23%); chronic pain syndrome (CPs: arthralgia and myalgia, 399, 22%); and neurosensorial syndrome (NSs: alteration in taste and smell, 197, 11%). Determinants of clinical phenotypes were different (all comparisons p < 0.05): being female increased risk of CPs, NSs, and CFs; chronic pulmonary diseases of REs; neurological symptoms at SARS-CoV-2 diagnosis of REs, NSs, and CFs; oxygen therapy of CFs and REs; and gastrointestinal symptoms at SARS-CoV-2 diagnosis of CFs. Early treatment of SARS-CoV-2 infection with monoclonal Ab (all clinical phenotypes), corticosteroids therapy for mild/severe cases (NSs), and SARS-CoV-2 vaccination (CPs) were less likely to be associated to PCS (all comparisons p < 0.05). Highest reduction in QoL was detected in REs and CPs (43.57 and 43.86 vs 57.32 in PCS-negative controls, p < 0.001). Female sex (p < 0.001), gastrointestinal symptoms (p = 0.034) and renal complications (p = 0.002) during the acute infection were likely to increase risk of severe PCS (QoL <50). Vaccination and early treatment with monoclonal Ab reduced the risk of severe PCS (p = 0.01 and p = 0.03, respectively). This study provides new evidence suggesting that PCS can be classified by clinical phenotypes with different impact on QoL, underlying possible different pathogenic mechanisms. Researchers identified factors associated to each clinical phenotype and to severe PCS. These results might help in designing pathogenesis studies and in selecting high-risk patients for inclusion in therapeutic and management clinical trials.
- Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality in older adults. In May 2023, the Food and Drug Administration approved the first two vaccines for prevention of RSV lower respiratory tract disease (LRTD) for use in adults aged ≥60 years. For both vaccine products, vaccination with a single RSV vaccine dose demonstrated moderate to high efficacy in preventing symptomatic RSV-associated LRTD among adults aged ≥60 years. On June 21, 2023, the Advisory Committee on Immunization Practices recommended that persons aged ≥60 years may receive a single dose of RSV vaccine, using shared clinical decision-making. RSV vaccination might prevent substantial morbidity in older adults at risk for severe RSV disease; postmarketing surveillance for safety and effectiveness will direct future guidance. - Viral Resistance Analyses From the Remdesivir Phase 3 Adaptive COVID-19 Treatment Trial-1 (ACTT-1)
Remdesivir is approved for the treatment of COVID-19 in non-hospitalized and hospitalized adult and pediatric patients. Here researchers present SARS-CoV-2 resistance analyses from the Phase 3 ACTT-1 randomized placebo-controlled trial conducted in adult participants hospitalized with COVID-19. Swab samples were collected at baseline and longitudinally through Day 29. SARS-CoV-2 genomes were sequenced using next-generation sequencing. Phenotypic analysis was conducted directly on participant virus isolates and/or using SARS-CoV-2 subgenomic replicons expressing mutations identified in the Nsp12 target gene. Among participants with both baseline and post-baseline sequencing data, emergent Nsp12 substitutions were observed in 12/31 (38.7%) and 12/30 (40.0%) participants in the remdesivir and placebo arms, respectively. No emergent Nsp12 substitutions in the remdesivir arm were observed in more than one participant. Phenotyping showed low to no change in susceptibility to remdesivir relative to wild-type Nsp12 reference for the substitutions tested: A16V (0.8-fold change in EC50), P323L+V792I (2.2-fold), C799F (2.5-fold), K59N (1.0-fold), and K59N+V792I (3.4-fold). The similar rate of emerging Nsp12 substitutions in remdesivir and placebo arms and the minimal change in remdesivir susceptibility among tested substitutions support a high barrier to remdesivir resistance development in COVID-19 patients. - Impact of Molnupiravir Treatment on Patient-Reported Coronavirus Disease 2019 (COVID-19) Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial
Molnupiravir is an orally administered antiviral authorized for COVID-19 treatment in adults at high risk of progression to severe disease. Here, researchers report secondary and post hoc analyses of participants’ self-reported symptoms in the MOVe-OUT trial, which evaluated molnupiravir initiated within 5 days of symptom onset in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed COVID-19. Eligible participants completed a 15-item symptom diary daily from day 1 (randomization) through day 29, rating symptom severity as “none,” “mild,” “moderate,” or “severe”; loss of smell and loss of taste were rated as “yes” or “no.” Time to sustained symptom resolution/improvement was defined as the number of days from randomization to the first of 3 consecutive days of reduced severity, without subsequent relapse. Time to symptom progression was defined as the number of days from randomization to the first of 2 consecutive days of worsening severity. The Kaplan-Meier method was used to estimate event rates at various time points. The Cox proportional hazards model was used to estimate the hazard ratio between molnupiravir and placebo. For most targeted COVID-19 symptoms, sustained resolution/improvement was more likely, and progression was less likely, in the molnupiravir versus placebo group through day 29. When evaluating 5 distinctive symptoms of COVID-19, molnupiravir participants had a shorter median time to first resolution (18 vs 20 d) and first alleviation (13 vs 15 d) of symptoms compared with placebo. Molnupiravir treatment in at-risk, unvaccinated patients resulted in improved clinical outcomes for most participant-reported COVID-19 symptoms compared with placebo.
- Predicting COVID-19 Incidence Using Wastewater Surveillance Data, Denmark, October 2021–June 2022
Analysis of wastewater is used in many settings for surveillance of SARS-CoV-2, but it remains unclear how well wastewater testing results reflect incidence. Denmark has had an extensive wastewater analysis system that conducts 3 weekly tests in ≈200 sites and has 85% population coverage; the country also offers free SARS-CoV-2 PCR tests to all residents. Using time series analysis for modeling, we found that wastewater data, combined with information on circulating variants and the number of human tests performed, closely fitted the incidence curve of persons testing positive. The results were consistent at a regional level and among a subpopulation of frequently tested healthcare personnel. Researchers used wastewater analysis data to estimate incidence after testing was reduced to a minimum after March 2022. These results imply that data from a large-scale wastewater surveillance system can serve as a good proxy for COVID-19 incidence and for epidemic control - Prevalence of Low-Frequency, Antiviral Resistance Variants in SARS-CoV-2 Isolates in Ontario, Canada, 2020-2023
This study included 78 866 clinical samples with next-generation whole-genome sequencing data for SARS-CoV-2. Low-frequency variants in the viral nsp5 gene were identified in 128 isolates (0.16%), and no single variant associated with antiviral resistance was predominate. This cohort study of low-frequency variants resistant to nirmatrelvir-ritonavir found that these variants were very rare in samples from patients with SARS-CoV-2, suggesting that selection of these variants by nirmatrelvir-ritonavir following the initiation of treatment may also be rare. Surveillance efforts that involve sequencing of viral isolates should continue to monitor for novel resistance variants as nirmatrelvir-ritonavir is used more broadly. - Relationship Between Azithromycin and Cardiovascular Outcomes in Unvaccinated Patients With COVID‐19 and Preexisting Cardiovascular Disease
This study was conducted using data from the ISACS‐COVID‐19 (International Survey of Acute Coronavirus Syndromes‐COVID‐19) registry. Patients with a confirmed diagnosis of SARS‐CoV‐2 infection were eligible for inclusion. The study included 793 patients exposed to azithromycin within 24 hours from hospital admission and 2141 patients who received only standard care. The primary exposure was cardiovascular disease (CVD). Main outcome measures were 30‐day mortality and acute heart failure (AHF). Among 2934 patients, 1066 (36.4%) had preexisting CVD. A total of 617 (21.0%) died, and 253 (8.6%) had AHF. Azithromycin therapy was consistently associated with an increased risk of AHF in patients with preexisting CVD (risk ratio [RR], 1.48 [95% CI, 1.06–2.06]). Receiving azithromycin versus standard care was not significantly associated with death (RR, 0.94 [95% CI, 0.69–1.28]). By contrast, we found significantly reduced odds of death (RR, 0.57 [95% CI, 0.42–0.79]) and no significant increase in AHF (RR, 1.23 [95% CI, 0.75–2.04]) in patients without prior CVD. The relative risks of death from the 2 subgroups were significantly different from each other (Pinteraction=0.01). Statistically significant association was observed between AHF and death (odds ratio, 2.28 [95% CI, 1.34–3.90]). - The effects of COVID-19 on cognitive performance in a community-based cohort: a COVID symptom study biobank prospective cohort study
Cognitive performance (working memory, attention, reasoning, motor control) was assessed in a prospective cohort study of participants from the United Kingdom COVID Symptom Study Biobank between July 12, 2021 and August 27, 2021 (Round 1), and between April 28, 2022 and June 21, 2022 (Round 2). Participants, recruited from the COVID Symptom Study smartphone app, comprised individuals with and without SARS-CoV-2 infection and varying symptom duration. Effects of COVID-19 exposures on cognitive accuracy and reaction time scores were estimated using multivariable ordinary least squares linear regression models weighted for inverse probability of participation, adjusting for potential confounders and mediators. The role of ongoing symptoms after COVID-19 infection was examined stratifying for self-perceived recovery. Longitudinal analysis assessed change in cognitive performance between rounds. 3335 individuals completed Round 1, of whom 1768 also completed Round 2. At Round 1, individuals with previous positive SARS-CoV-2 tests had lower cognitive accuracy (N = 1737, β = −0.14 standard deviations, SDs, 95% confidence intervals, CI: −0.21, −0.07) than negative controls. Deficits were largest for positive individuals with ≥12 weeks of symptoms (N = 495, β = −0.22 SDs, 95% CI: −0.35, −0.09). Effects were comparable to hospital presentation during illness (N = 281, β = −0.31 SDs, 95% CI: −0.44, −0.18), and 10 years age difference (60–70 years vs. 50–60 years, β = −0.21 SDs, 95% CI: −0.30, −0.13) in the whole study population. Stratification by self-reported recovery revealed that deficits were only detectable in SARS-CoV-2 positive individuals who did not feel recovered from COVID-19, whereas individuals who reported full recovery showed no deficits. Longitudinal analysis showed no evidence of cognitive change over time, suggesting that cognitive deficits for affected individuals persisted at almost 2 years since initial infection. Cognitive deficits following SARS-CoV-2 infection were detectable nearly two years post infection, and largest for individuals with longer symptom durations, ongoing symptoms, and/or more severe infection. However, no such deficits were detected in individuals who reported full recovery from COVID-19. Further work is needed to monitor and develop understanding of recovery mechanisms for those with ongoing symptoms.
Situation Dashboards



World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)


Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU


COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources


Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.