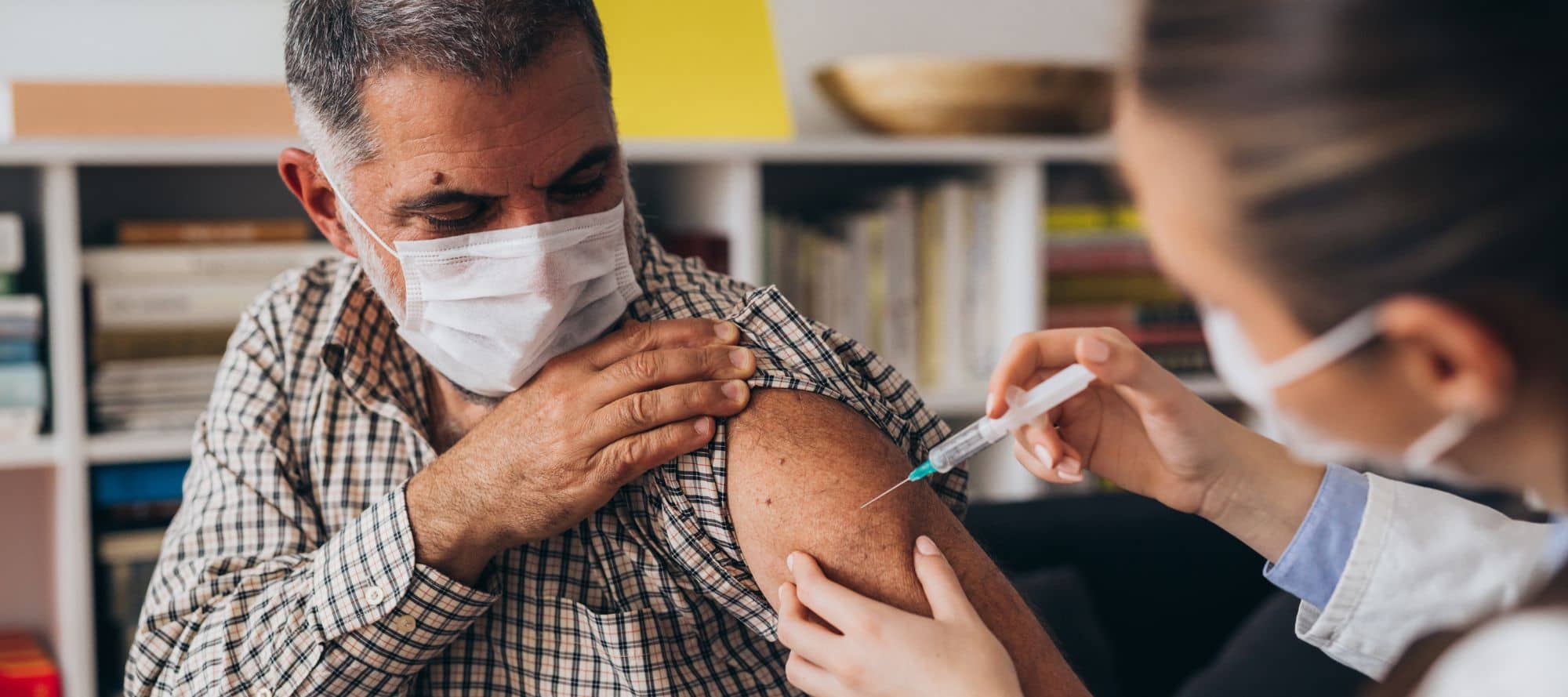
- Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons
These are the results of a phase 3, blinded trial, where ≥60-year-olds were randomized (1:1) to receive RSVPreF3 OA or placebo pre-season 1. Then they looked at season 2 and the potential need for revaccination. They took the previously vaccinated individuals and re-randomized them (1:1) to receive a second RSVPreF3 OA dose (RSV revaccination group) or placebo (RSV_1dose group) pre-season 2; Efficacy and safety of the vaccine regimens against RSV-LRTD was evaluated over two seasons. The efficacy analysis comprised 24,967 participants (RSV_1dose: 6227, RSV revaccination: 6,242, placebo: 12,498). The median efficacy follow-up was 17.8 months. Efficacy over 2 seasons of 1 RSVPreF3 OA dose was 67.2% (97.5% CI: 48.2-80.0) against RSV-LRTD and 78.8% (95% CI: 52.6-92.0) against severe RSV-LRTD. Efficacy over two seasons of a first dose followed by revaccination was 67.1% (97.5% CI: 48.1-80.0) against RSV-LRTD and 78.8% (95% CI: 52.5-92.0) against severe RSV-LRTD. So, no benefit to revaccination after one year. Reactogenicity/safety of the revaccination dose were similar to dose 1.
- Effectiveness of High-dose vs. Standard-dose Quadrivalent Influenza Vaccine against Recurrent Hospitalizations and Mortality in Relation to Influenza Circulation: A Post-hoc Analysis of the DANFLU-1 Randomized Clinical Trial
Analysis compared the relative effectiveness of high-dose quadrivalent influenza vaccine (QIV-HD) vs. standard-dose quadrivalent influenza vaccine (QIV-SD) against hospitalization and found that among 12,477 randomly assigned participants, receiving QIV-HD was associated with lower incidence rates of hospitalizations for pneumonia or influenza (10 vs. 33 events, IRR 0.30 [95% CI 0.14-0.64], p=0.002) and all-cause hospitalizations (647 vs. 742 events, IRR 0.87 [95% CI 0.76-0.99], p=0.032) compared with QIV-SD. Trends favoring QIV-HD were consistently observed over time. They concluded with “Our exploratory results correspond to a number needed to treat of 65 (95% CI 35-840) persons vaccinated with QIV-HD compared with QIV-SD to prevent one additional all-cause hospitalization per season. Further research is needed to confirm these hypothesis-generating findings.”
- Changes in Markers of Inflammation and Their Correlation with Death in Patients with COVID-19 in the Intensive Care Unit
Authors looked at 58 participants: i) inpatients (n = 37): patients suffering from severe acute respiratory syndrome due to COVID-19, who were admitted at Intensive Care Unit (ICU) [some recovered and some died] and ii) control group (n = 21): community volunteers. They looked at interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-17 (IL-17), interferon-gamma (IFN-γ) and some other parameters such as levels of Urea, LDH, D-dimer, PT/INR, AST, ALT and lymphocytes. Confirmatory in that they found that high levels of inflammatory markers, such as pro-inflammatory cytokines, and laboratory parameters, such as low levels of lymphocytes (remember that NLR) and high levels of IL-6, were associated with disease severity, especially in individuals who died.
- Long COVID is Associated with Severe Cognitive Slowing: A Multicentre Cross-sectional Study
Patients with PCC completed two short web-based cognitive tasks, Simple Reaction Time (SRT) and Number Vigilance Test (NVT). 270 patients diagnosed with post-COVID-19 conditions, (PCC), at two different clinics in UK and Germany were compared to two control groups: individuals who contracted COVID-19 before but did not experience PCC after recovery (No-PCC group) and uninfected individuals (No-COVID group). All patients with PCC completed the study between May 18, 2021 and July 4, 2023 in Jena University Hospital, Jena, Germany and Long COVID clinic, Oxford, UK. They identified pronounced cognitive slowing in patients with PCC, which distinguished them from age-matched healthy individuals who previously had symptomatic COVID-19 but did not manifest PCC. Cognitive slowing was evident even on a 30-s task measuring simple reaction time (SRT), with patients with PCC responding to stimuli ∼3 standard deviations slower than healthy controls. 53.5% of patients with PCC's response speed was slower than 2 standard deviations from the control mean, indicating a high prevalence of cognitive slowing in PCC. This finding was replicated across two clinic samples in Germany and the UK. Comorbidities such as fatigue, depression, anxiety, sleep disturbance, and post-traumatic stress disorder did not account for the extent of cognitive slowing in patients with PCC. Furthermore, cognitive slowing on the SRT was highly correlated with the poor performance of patients with PCC on the NVT measure of sustained attention.
Situation Dashboards

World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)

Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU

COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources

Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.