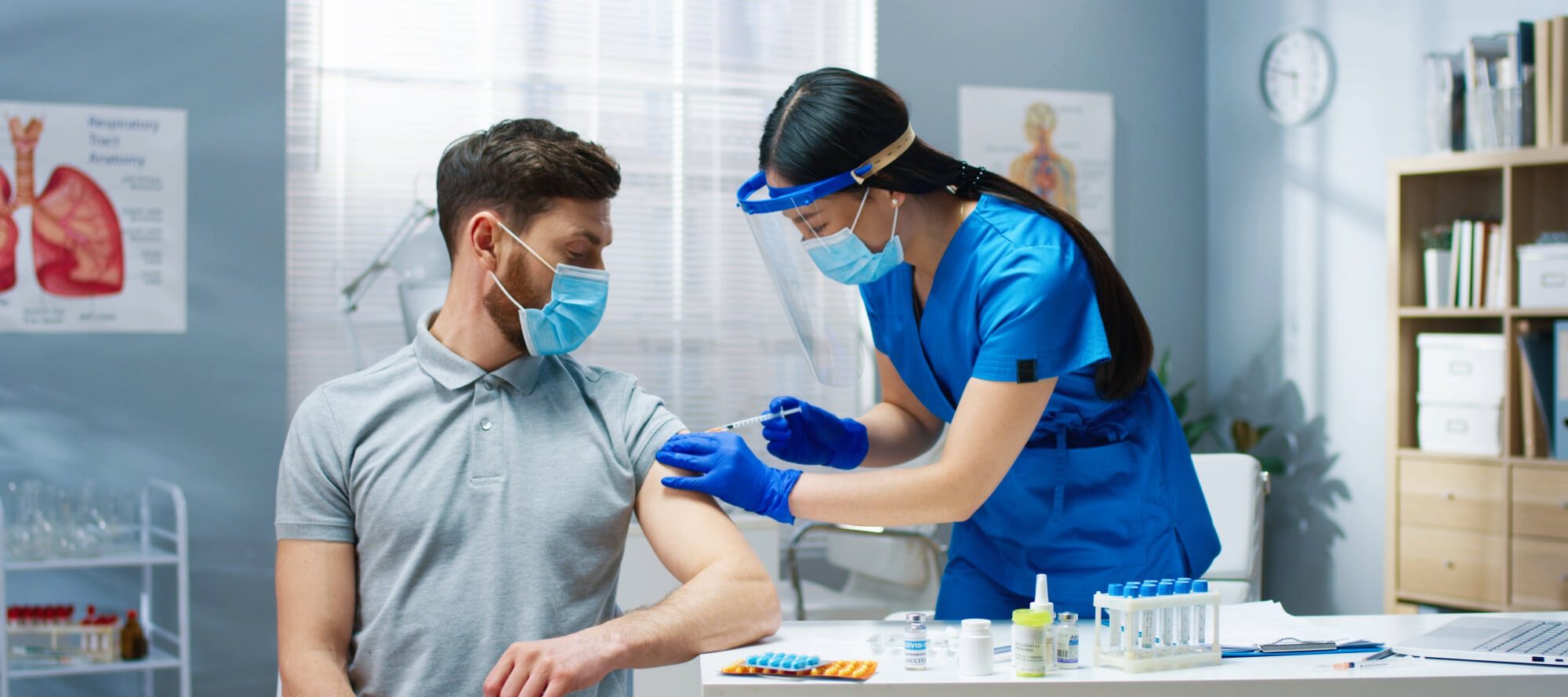
- Mpox Vaccination Hesitancy, Previous Immunization Coverage, and Vaccination Readiness in the African Region: A Multinational Survey
Authors findings indicate a significant level of hesitancy toward mpox vaccination in the African region. Individuals who have not previously received other vaccines are at a higher risk of refusing to vaccinate against mpox for themselves and for children. However, high vaccination readiness can help mitigate this risk. The study recommends that regions in Africa with low immunization coverage should continue to enhance vaccination education and improve vaccination readiness to reduce hesitancy and promote the mpox vaccination program - Global Prevalence and Correlates of Mpox Vaccine Acceptance and Uptake: A Systematic Review and Meta-analysis
Study searched multiple databases for peer-reviewed studies published in English from May 2022 to 25th November 2023 that evaluated mpox vaccine acceptance and/or uptake. Authors fit a random-effects model meta-analysis to calculate the pooled mpox vaccine acceptance and uptake rates, with their 95% confidence intervals (CI) across population outcomes. Subgroup analyses were performed among the six World Health Organization (WHO) regions (Africa [AFR], Region of the Americas [AMR], South-East Asia Region [SEAR], European Region [EUR], Eastern Mediterranean Region [EMR], and the Western Pacific Region [WPR]), as well as among select population subgroups. Conclusion: Tailored interventions are needed to bolster confidence in the mpox vaccine, maximize vaccine uptake, and increase vaccine access to close the gaps between acceptance and uptake especially among key populations residing in regions with low rates of acceptance and uptake
- The Effect of Nirmatrelvir/ritonavir on Short- and Long-term Adverse Outcomes from COVID-19 among Patients with Kidney Disease: A Propensity-score Matched Study
Here 1,095 nirmatrelvir-ritonavir-treated patients were matched to 584 comparators. Patients who received nirmatrelvir-ritonavir patients were less likely to be hospitalized within 30 days of diagnosis (adjusted subdistribution hazard ratio [sHR] 0.44, confidence interval [CI] 0.26–0.73, p<0.01). At one year, nirmatrelvir-ritonavir-treated patients had lower risk of hospitalization for MACE (adjusted sHR 0.49 [0.36–0.67], p<0.01) and death (adjusted hazard ratio [aHR] 0.37 [0.21–0.65], p<0.01). Use of nirmatrelvir-ritonavir was not associated with decreased risk of CKD progression or attenuation of eGFR decline slope in the year following infection. In an associated CIDRAP post they point out that 94% of patients had pre-dialysis CKD, and 6% had kidney failure. Nearly all (92%) had been vaccinated against COVID-19, and 2% previously had COVID-19. Patients given Paxlovid were significantly less likely than comparators to be hospitalized within 30 days of diagnosis (3% vs 8%; adjusted subdistribution hazard ratio [sHR], 0.44) and less likely to die (0% vs 3%)
- Epidemiological Insights into Chronic Urticaria, Vitiligo, Alopecia Areata, and Herpes Zoster following COVID-19 Infection: A Nationwide Population-based Study
This study aimed to estimate the incidence and risk of chronic urticaria, vitiligo, alopecia areata, and herpes zoster following COVID-19 infection. Only participants confirmed by real-time reverse transcription-polymerase chain reaction tests to have COVID-19 were enrolled in the COVID-19 group. The matched cohort without COVID-19 was enrolled randomly at a ratio of 1:1. The incidence and risk of chronic urticaria, vitiligo, alopecia areata, and herpes zoster were assessed in both groups using univariable and multivariable Cox proportional hazard analyses. A total of 4 976 589 COVID-19 patients (9.58% of the total population of South Korea) and an equivalent number of matched non-infected control subjects were analyzed. Chronic urticaria, vitiligo, alopecia areata, and herpes zoster manifested at higher rates within the COVID-19 cohort, even after multivariable adjustment for potential confounders. - Impact of Extended-course Oral Nirmatrelvir/ritonavir in Established Long COVID: A Case Series
This is a shared case series of 13 individuals with Long COVID who initiated extended courses (>5 days; range: 7.5–30 days) of oral nirmatrelvir/ritonavir outside (n = 11) of and within (n = 2) the context of an acute SARS-CoV-2 infection. Participants reported on symptoms and health experiences before, during, and after their use of nirmatrelvir/ritonavir.
Situation Dashboards

World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)

Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU

COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources

Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.