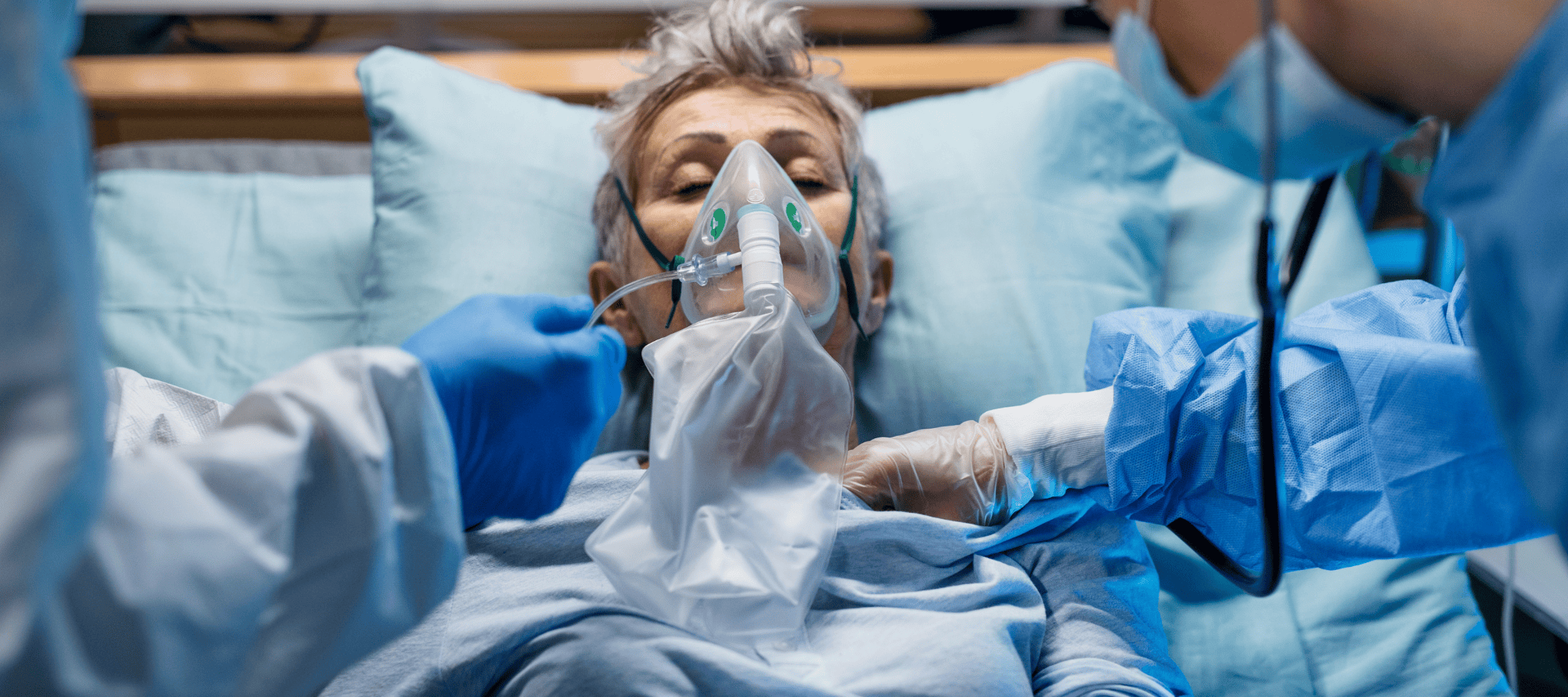
- Tecovirimat Use under Expanded Access to Treat Mpox in the United States, 2022–2023
Tecovirimat was prescribed for over 7,100 patients in the United States, most often for lesions in sensitive anatomical areas, such as certain anogenital lesions (83.5%; 5135 out of 6148 patients), and pain (52.5%; 3227 out of 6148 patients). The demographic and clinical characteristics mirrored those of patients worldwide. Among the 7,181 patients with returned intake forms, 1,626 also had returned outcome forms (22.6%). Many patients with severe immunocompromise (e.g., HIV with CD4 counts <200 cells/μl) received multiple courses of tecovirimat (43.1%; 22 out of 51 patients), including intravenously, and often experienced poor outcomes (35.3%; 18 out of 51 patients). Overall, 223 SAEs and 40 deaths were reported. Most SAEs were among patients who were severely immunocompromised, one of whom experienced hallucinations after tecovirimat was administered at twice the standard dose.
- Effectiveness of Updated 2023–2024 (Monovalent XBB.1.5) COVID-19 Vaccination Against SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 Lineage Hospitalization and a Comparison of Clinical Severity—IVY Network, 26 Hospitals, 18 October 2023–9 March 2024
Investigators analyzed patients hospitalized with COVID-19–like illness at 26 hospitals in 20 U.S. states admitted 18 October 2023 to 9 March 2024. Using a test-negative, case-control design, they estimated effectiveness of an updated 2023–2024 (monovalent XBB.1.5) COVID-19 vaccine dose against sequence-confirmed XBB and JN lineage hospitalization. Odds of severe outcomes, including intensive care unit (ICU) admission and invasive mechanical ventilation (IMV) or death, were compared for JN versus XBB lineage hospitalizations. A total of 585 case-patients with XBB lineages, 397 case-patients with JN lineages, and 4,580 control patients were included. VE in the first seven to 89 days after receipt of an updated dose was 54.2% (95% confidence interval [CI], 36.1–67.1%) against XBB lineage hospitalization and 32.7% (95% CI, 1.9–53.8%) against JN lineage hospitalization. Odds of ICU admission (adjusted odds ratio [aOR], .80; 95% CI, .46–1.38) and IMV or death (aOR, .69; 95% CI, .34–1.40) were not significantly different among JN compared with XBB lineage hospitalizations. The authors point out that, “ These findings indicate that, despite substantial genomic divergence of JN lineages from XBB.1.5, updated COVID-19 vaccines continued to provide protection against COVID-19–associated hospitalization. Wide CIs for some estimates precluded confident assessment of the extent to which VE differs by lineage.” - Early, Robust Mucosal Secretory Immunoglobulin A but not Immunoglobulin G Response to Severe Acute Respiratory Syndrome Coronavirus 2 Spike in Oral Fluid Is Associated With Faster Viral Clearance and Coronavirus Disease 2019 Symptom Resolution
Authors investigated the role of oral mucosal antibody responses in viral clearance and COVID-19 symptom duration. (IgA is the main mucosal antibody type as opposed to IgG that is the main serum type.) They gave participants with PCR-confirmed SARS-CoV-2 infection oral fluid for testing with SARS-CoV-2 antibody multiplex assays, nasal swabs for RT-PCR and symptom information at up to eight follow-ups from April 2020 to February 2022. They found that high and moderate oral fluid anti-spike (S) secretory IgA (SIgA) post infection was associated with significantly faster viral clearance and symptom resolution across age groups with effect sizes equivalent to having COVID-19 vaccine immunity at the time of infection. Those with high and moderate anti-S SIgA cleared the virus 14 days (95% CI: 10-18) and recovered 9-10 days (95% CI: 6-14) earlier. Delayed and higher anti-S IgG was associated with significantly longer time to clearance and recovery. Experiencing symptoms longer than four weeks was associated with lower anti-RBD SIgA 15-30 days after infection onset (p<0.001).
- Prevalent Metformin Use in Adults With Diabetes and the Incidence of Long COVID: An EHR-Based Cohort Study From the RECOVER Program
These are the results of a retrospective cohort analysis using the National COVID Cohort Collaborative (N3C) and Patient-Centered Clinical Research Network (PCORnet) electronic health record (EHR) databases where they looked at individuals with Type 2 DM who were or who were not taking metformin when diagnosed with COVID-19. Kind of mixed depending on which database they look at. Just looking at the NC3 data we get a suggested 15-21% decrease in PASC and looking that the PCORnet database it might be decreased by 13% or increased by 4% but overlapping confidence intervals with no change. Looking at raw percentages we might be talking about numbers down around 1% or so in terms of these differences.
Situation Dashboards

World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)

Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU

COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources

Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.