- Clinical course of SARS-CoV-2 infection and recovery in lung transplant recipients
Reports on outcomes following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in lung transplant recipients remain limited. Researchers performed a single-center, observational study of outcomes in lung transplant recipients diagnosed with SARS-CoV-2 between 5/1/2020 and 3/15/2022 that were followed for a median of 123 days. Researchers analyzed changes in spirometry, acute lung allograft dysfunction (ALAD) incidence, hospitalization, mechanical ventilation needs, secondary infection, and survival. In this cohort of 336 patients, 103 developed coronavirus disease (COVID) (27 pre-Delta, 20 Delta, and 56 Omicron-era). Twenty-five patients (24%) required hospitalization and 10 patients ultimately died (10%). Among 85 survivors who completed ambulatory spirometry, COVID-19 did not alter change in forced expiratory volume in 1 s (FEV1) or forced vital capacity (FVC) over time compared to the preceding 6 months. The pre-COVID FEV1change was −0.05 ml/day (IQR −0.50 to 0.60) compared to −0.20 ml/day (IQR −1.40 to 0.70) post-COVID (p = .16). The pre-COVID change in FVC was 0.20 ml/day (IQR −0.60 to 0.70) compared to 0.05 ml/day (IQR −1.00 to 1.10) post-COVID (p = .76). Although the cohort overall had stable lung function, 33 patients (39%) developed ALAD or accelerated chronic lung allograft dysfunction (FEV1 decline >10% from pre-COVID baseline). Nine patients (35%) with ALAD recovered lung function. Within 3 months of acute COVID infection, 18 patients (17%) developed secondary infections, the majority being bacterial pneumonia. Finally, vaccination with at least two doses of mRNA vaccine was not associated with improved outcomes. This study describes the natural history of SARS-CoV-2 infection in a large cohort of lung transplant recipients. Although one third of patients develop ALAD requiring augmented immunosuppression, infection with SARS-CoV-2 is not associated with worsening lung function - Acute and postacute sequelae associated with SARS-CoV-2 reinfection
First infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with increased risk of acute and postacute death and sequelae in various organ systems. Whether reinfection adds to risks incurred after first infection is unclear. Here researchers used the US Department of Veterans Affairs’ national healthcare database to build a cohort of individuals with one SARS-CoV-2 infection (n = 443,588), reinfection (two or more infections, n = 40,947) and a noninfected control (n = 5,334,729). Researchers used inverse probability-weighted survival models to estimate risks and 6-month burdens of death, hospitalization and incident sequelae. Compared to no reinfection, reinfection contributed additional risks of death (hazard ratio (HR) = 2.17, 95% confidence intervals (CI) 1.93–2.45), hospitalization (HR = 3.32, 95% CI 3.13–3.51) and sequelae including pulmonary, cardiovascular, hematological, diabetes, gastrointestinal, kidney, mental health, musculoskeletal and neurological disorders. The risks were evident regardless of vaccination status. The risks were most pronounced in the acute phase but persisted in the postacute phase at 6 months. Compared to noninfected controls, cumulative risks and burdens of repeat infection increased according to the number of infections. Limitations included a cohort of mostly white males. The evidence shows that reinfection further increases risks of death, hospitalization and sequelae in multiple organ systems in the acute and postacute phase. Reducing overall burden of death and disease due to SARS-CoV-2 will require strategies for reinfection prevention. - Cognitive Deficits in Long Covid-19
The finding of axonal demyelination (or impaired myelination) in sections of mouse brain could inspire the development of new magnetic resonance imaging biomarkers for humans. It should be noted, however, that Fernández-Castañeda et al. used the earliest strain of SARS-CoV-2 (known as the original Wuhan-Hu-1 isolate or USA-WA1/2020); the relevance of their findings to brain fog associated with infection by other SARS-CoV-2 variants seems likely but uncertain. Moreover, as the authors themselves noted, the contribution of other cell types, such as astrocytes, to Covid-related brain fog may be substantive. Finally, there is the usual caveat that mice are not humans, so these findings warrant robust tests of replication in studies involving a larger number of patients. Although the findings of brain dysfunction and patterns of damage during and after Covid are worrisome, especially given the similarities with changes in human neurodegenerative diseases, translational studies such as the one reported by Fernández-Castañeda may point to paths toward accurate diagnoses and treatments. - Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: a prospective cross-sectional study
Growing evidence links COVID-19 with acute and long-term neurological dysfunction. However, the pathophysiological mechanisms resulting in central nervous system involvement remain unclear, posing both diagnostic and therapeutic challenges. Here researchers show outcomes of a cross-sectional clinical study (NCT04472013) including clinical and imaging data and corresponding multidimensional characterization of immune mediators in the cerebrospinal fluid (CSF) and plasma of patients belonging to different Neuro-COVID severity classes. The most prominent signs of severe Neuro-COVID are blood-brain barrier (BBB) impairment, elevated microglia activation markers and a polyclonal B cell response targeting self-antigens and non-self-antigens. COVID-19 patients show decreased regional brain volumes associating with specific CSF parameters, however, COVID-19 patients characterized by plasma cytokine storm are presenting with a non-inflammatory CSF profile. Post-acute COVID-19 syndrome strongly associates with a distinctive set of CSF and plasma mediators. Collectively, researchers identify several potentially actionable targets to prevent or intervene with the neurological consequences of SARS-CoV-2 infection.
- Tecovirimat is effective against human monkeypox virus in vitro at nanomolar concentrations
The ongoing monkeypox virus (MPXV) outbreak is the largest ever recorded outside of Africa. Researchers isolated and sequenced a virus from the first clinical MPXV case diagnosed in France (May 2022). They report that tecovirimat (ST-246), a US Food and Drug Administration approved drug, is efficacious against this isolate in vitro at nanomolar concentrations, whereas cidofovir is only effective at micromolar concentrations. Our results support the use of tecovirimat in ongoing human clinical trials. - Effectiveness of a third BNT162b2 mRNA COVID-19 vaccination during pregnancy: a national observational study in Israel
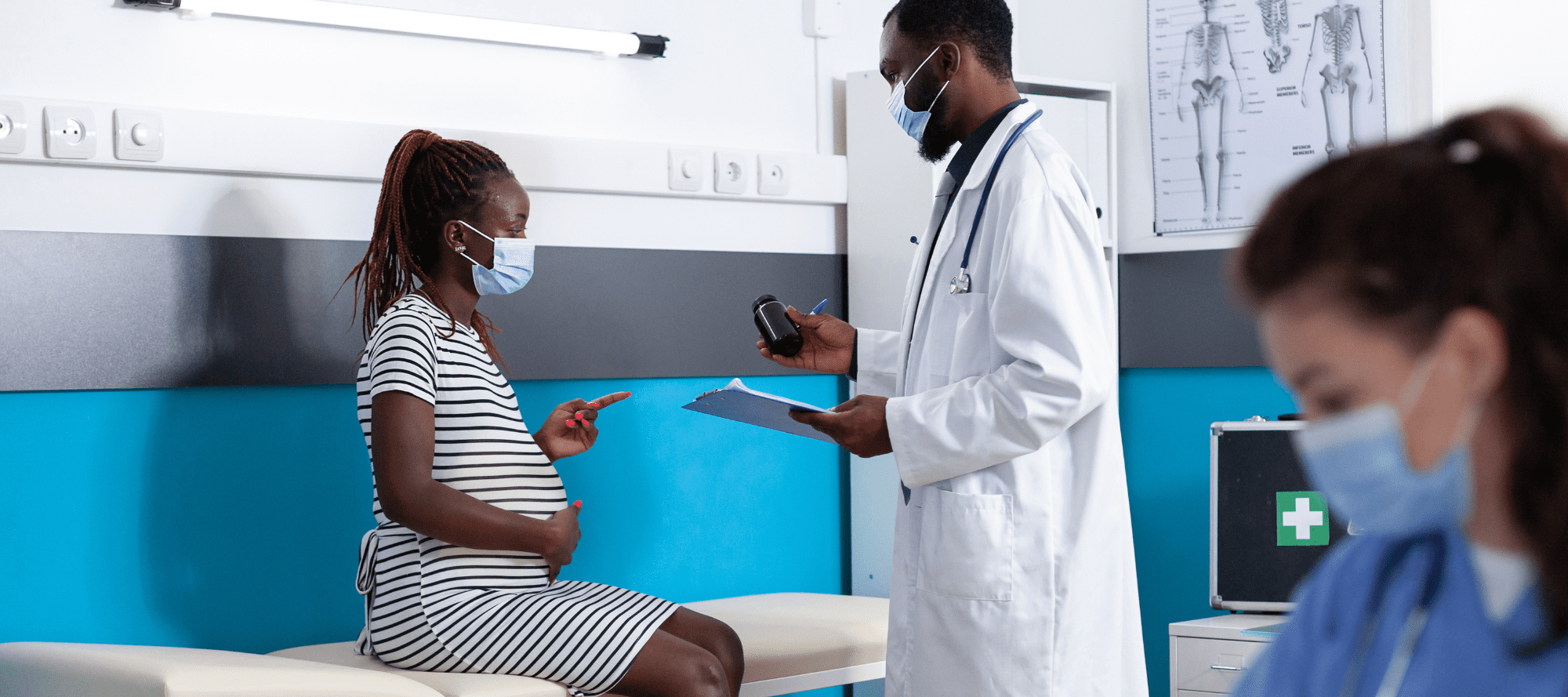
The Centers for Disease Control (CDC) recommend a third dose of COVID-19 vaccine for pregnant women, although data regarding effectiveness during pregnancy are lacking. This national, population-based, historical cohort study of pregnant women in Israel, delivering between August 1, 2021 and March 22, 2022, aims to analyze and compare the third and second doses’ vaccine effectiveness in preventing COVID-19-related hospitalizations during pregnancy during two COVID-19 waves (Delta variant in the summer of 2021 and Omicron, BA.1, variant in the winter of 2022). Time-dependent Cox proportional-hazards regression models estimate the hazard ratios (HR) and 95% confidence intervals (CI) for COVID-related outcomes according to vaccine dose, and vaccine effectiveness as 1-HR. Study includes 82,659 and 33,303 pregnant women from the Delta and Omicron waves, respectively. Compared with the second dose, the third dose effectively prevents overall hospitalizations with SARS-CoV-2 infections, with estimated effectiveness of 92% (95% CI 83–96%) during Delta, and enhances protection against significant disease during Omicron, with effectiveness of 92% (95% CI 26–99%), and 48% (95% CI 37–57%) effectiveness against hospitalization overall. A third dose of the BNT162b2 mRNA COVID-19 vaccine during pregnancy, given at least 5 months after the second vaccine dose, enhances protection against adverse COVID-19-related outcomes. - Maternal Antibody Response and Transplacental Transfer Following Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection or Vaccination in Pregnancy
COVID-19 vaccination in pregnancy leads to higher and longer lasting maternal IgG levels, higher cord blood IgG, and higher transfer ratio after 90 days compared with SARS-CoV-2 infection. Greater infection severity leads to higher maternal and cord blood antibodies. Maternal IgG decreases over time following both vaccination and infection, reinforcing the importance of vaccination, even after infection, and vaccine boosters for pregnant patients. - Systematic review of the clinical effectiveness of Tixagevimab/Cilgavimab for prophylaxis of COVID-19 in immunocompromised patients
There is a growing body of real-world evidence validating the original PROVENT phase III study regarding the clinical effectiveness of Tixagevimab/Cilgavimab as prophylaxis for immunocompromised patients, notably demonstrating effectiveness during the Omicron wave. This review demonstrates the clinical effectiveness of prophylactic Tixagevimab/Cilgavimab at reducing COVID-19 infection, hospitalization, ITU admission and mortality for immunosuppressed individuals. It is important that ongoing larger-scale and better-controlled real world studies are initiated and evaluated to provide ongoing certainty of the clinical benefit of prophylactic antibody treatment for immunocompromised patients in the face of new variants. - Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform
Between 16 December 2021 and 10 February 2022, 3331 and 2689 patients were treated with sotrovimab and molnupiravir, respectively, with no substantial differences in baseline characteristics. Mean age of all 6020 patients was 52 (standard deviation 16) years; 59% were women, 89% were white, and 88% had received three or more covid-19 vaccinations. Within 28 days of the start of treatment, 87 (1.4%) patients were admitted to hospital or died of infection from SARS-CoV-2 (32 treated with sotrovimab and 55 with molnupiravir). Cox proportional hazards models stratified by area showed that after adjusting for demographic information, high risk cohort categories, vaccination status, calendar time, body mass index, and other comorbidities, treatment with sotrovimab was associated with a substantially lower risk than treatment with molnupiravir (hazard ratio 0.54, 95% confidence interval 0.33 to 0.88, P=0.01). Consistent results were found from propensity score weighted Cox models (0.50, 0.31 to 0.81, P=0.005) and when restricted to people who were fully vaccinated (0.53, 0.31 to 0.90, P=0.02). No substantial effect modifications by other characteristics were detected (all P values for interaction >0.10). The findings were similar in an exploratory analysis of patients treated between 16 February and 1 May 2022 when omicron BA.2 was the predominant variant in England. In routine care of adult patients in England with covid-19 in the community, at high risk of severe outcomes from covid-19, those who received sotrovimab were at lower risk of severe outcomes of covid-19 than those treated with molnupiravir. - The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants
The uptake of Paxlovid in individuals infected with COVID-19 has been significantly limited by concerns around the Paxlovid rebound phenomenon despite the scarcity of evidence around its epidemiology. The purpose of this study was to prospectively compare the epidemiology of Paxlovid rebound in treated and untreated participants with acute COVID-19 infection Methods: Researchers designed a digital, prospective observational study, which included participants who tested positive for COVID-19 and were clinically eligible for Paxlovid. Participants were assigned to a Paxlovid or control group based on their decision to take the medication. Both groups were provided 12 rapid antigen tests and asked to test and answer symptom surveys on a regular frequent schedule for 16 days. Viral rebound based on test results and COVID-19 symptom rebound based on patient reported symptoms were evaluated. Results: Viral rebound incidence was 14.2% in the Paxlovid group (n=127) and 9.3% in the control group (n=43). COVID-19 symptom rebound incidence was higher in the Paxlovid group (18.9%) compared to the control group (7.0%). There were no notable differences in viral rebound by age, gender, pre-existing conditions, or major symptom groups during the acute phase or at the 1-month interval. Conclusion: This preliminary report of our prospective study suggests that rebound after clearance of test positivity or symptom resolution is higher than previously reported. However, researchers observed a similar rate of rebound in both in the Paxlovid and control groups. Large studies with diverse participants and extended follow-up are needed to better understand the rebound phenomena. - Monoclonal Antibodies for Treatment of SARS-CoV-2 Infection During Pregnancy
In pregnant persons with mild to moderate COVID-19, adverse events after mAb treatment were mild and rare. There was no difference in obstetric-associated safety outcomes between mAb treatment and no treatment among persons who delivered. There was no difference in 28-day COVID-19–associated outcomes and non-COVID-19–related hospital admissions for mAb treatment compared with no mAb treatment in a propensity score–matched cohort. - Twice daily oral zinc in the treatment of patients with Coronavirus Disease-19 A randomized double-blind controlled trial
190 patients (40.4%) were ambulatory and 280 patients (59.6%) were hospitalized. Mortality at 30 days was 6.5% in the zinc group and 9.2% in the placebo group (OR: .68; 95% CI .34–1.35); ICU admission rates were, respectively, 5.2% and 11.3% (OR: .43; 95% CI .21–.87). Combined outcome was lower in the zinc group versus the placebo group (OR: .58; 95% CI .33–.99). Consistent results were observed in prespecified subgroups of patients aged <65 years, those with comorbidity, and those who needed oxygen therapy at baseline. Length of hospital stay was shorter in the zinc group versus the placebo group (difference: 3.5 days; 95% CI 2.76–4.23) in the inpatient group; duration of COVID-19 symptoms decreased with zinc treatment versus placebo in outpatients (difference: 1.9 days; 95% CI .62–2.6). No severe adverse events were observed during the study. These results showed that, in COVID-19 patients, oral zinc can decrease 30-day death, ICU admission rate and can shorten symptom duration.
- The diagnostic accuracy of rapid diagnostic tests for Ebola virus disease: a systematic review
Summary estimates of diagnostic accuracy study were produced for each device type. Subgroup analyses were performed for RDT type and specimen material. A sensitivity analysis was performed to assess the effect of trial design and bias. Researchers included 15 diagnostic accuracy studies. The summary estimate of sensitivity for lateral flow assays was 86.1% (95% CI, 86–86.2%), with specificity of 97% (95% CI, 96.1–97.9%). The summary estimate for rapid PCR devices was sensitivity of 96.2% (95% CI, 95.3–97.9%), with a specificity of 96.8% (95% CI, 95.3–97.9%). Pre-specified subgroup analyses demonstrated that RDTs were effective on a range of specimen material. Overall, the risk of bias throughout the included studies was low, but it was high in patient selection and uncertain in the flow and timing domains. RDTs possess both high sensitivity and specificity compared with RT-PCR among symptomatic patients presenting to the Ebola treatment units. These findings support the use of RDTs as a ‘rule in’ test to expedite treatment and vaccination. - Evaluating the accuracy of self-collected swabs for the diagnosis of monkeypox
Researchers evaluated the accuracy of patient-collected skin lesions, oropharyngeal, and rectal swabs amongst 50 individuals enrolled in a study of monkeypox viral dynamics. They found that the performance of self-collected samples was similar to that of physician-collected samples, suggesting that self-sampling is a reliable strategy for diagnosing monkeypox.
- The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study
Respiratory syncytial virus (RSV) is a major cause of hospitalization in infants. The burden of RSV infection in healthy term infants has not yet been established. Accurate health-care burden data in healthy infants are necessary to determine RSV immunization policy when RSV immunization becomes available. Researchers performed a multicenter, prospective, observational birth cohort study in healthy term-born infants (≥37 weeks of gestation) in five sites located in different European countries to determine the health-care burden of RSV. The incidence of RSV-associated hospitalizations in the first year of life was determined by parental questionnaires and hospital chart reviews. Researchers performed active RSV surveillance in a nested cohort to determine the incidence of medically attended RSV infections. In total, 9154 infants born between July 1, 2017, and April 1, 2020, were followed up during the first year of life and 993 participated in the nested active surveillance cohort. The incidence of RSV-associated hospitalizations in the total cohort was 1·8% (95% CI 1·6–2·1). There were eight pediatric intensive care unit admissions, corresponding to 5·5% of 145 RSV-associated hospitalizations and 0·09% of the total cohort. Incidence of RSV infection in the active surveillance cohort confirmed by any diagnostic assay was 26·2% (24·0–28·6) and that of medically attended RSV infection was 14·1% (12·3–16·0). RSV-associated acute respiratory infection causes substantial morbidity, leading to the hospitalization of one in every 56 healthy term-born infants in high-income settings. Immunization of pregnant women or healthy term-born infants during their first winter season could have a major effect on the health-care burden caused by RSV infections. - Impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh
A randomized-trial of community-level mask promotion in rural Bangladesh during the COVID-19 pandemic shows that the intervention increased mask usage and reduced symptomatic SARS-CoV-2 infections, demonstrating that promoting community mask-wearing can improve public health. - Lifting Universal Masking in Schools — Covid-19 Incidence among Students and StaffBefore the statewide masking policy was rescinded, trends in the incidence of Covid-19 were similar across school districts. During the 15 weeks after the statewide masking policy was rescinded, the lifting of masking requirements was associated with an additional 44.9 cases per 1000 students and staff (95% confidence interval, 32.6 to 57.1), which corresponded to an estimated 11,901 cases and to 29.4% of the cases in all districts during that time. Districts that chose to sustain masking requirements longer tended to have school buildings that were older and in worse condition and to have more students per classroom than districts that chose to lift masking requirements earlier. In addition, these districts had higher percentages of low-income students, students with disabilities, and students who were English-language learners, as well as higher percentages of Black and Latinx students and staff. Our results support universal masking as an important strategy for reducing Covid-19 incidence in schools and loss of in-person school days. As such, we believe that universal masking may be especially useful for mitigating effects of structural racism in schools, including potential deepening of educational inequities. Among school districts in the greater Boston area, the lifting of masking requirements was associated with an additional 44.9 Covid-19 cases per 1000 students and staff during the 15 weeks after the statewide masking policy was rescinded.
- Early Adoption of Anti–SARS-CoV-2 Pharmacotherapies Among US Veterans With Mild to Moderate COVID-19, January and February 2022
In this cohort study of 111 717 outpatient US veterans with clinical risk factors for severe COVID-19 who tested positive for SARS-CoV-2 during January and February 2022, 4233 (3.8%) received outpatient pharmacotherapy. Black veterans and Hispanic veterans were less likely to receive treatment, whereas older veterans with a higher number of underlying conditions were more likely to receive treatment. These findings suggest that during a 2-month period when 4 anti–SARS-CoV-2 pharmacotherapies were authorized for use, few eligible veterans received treatment. - Long-lasting Symptoms After an Acute COVID-19 Infection and Factors Associated With Their Resolution
A total of 3972 participants (2531 women [63.7%; 95% CI, 62.2%-65.2%]; mean [SD] age, 50.9 [12.7] years) had been infected with SARS-CoV-2. Of these 3972 participants, 2647 (66.6% [95% CI, 65.1%-68.1%]) reported at least 1 symptom during the acute phase. Of these 2647 participants, 861 (32.5% [95% CI, 30.8%-34.3%]) reported at least 1 persistent symptom lasting 2 or more months after the acute phase. After 1 year of follow-up, the estimated proportion of individuals with complete symptom resolution was 89.9% (95% CI, 88.7%-90.9%) with acute symptoms. Older age (>60 years; HR, 0.78; 95% CI, 0.68-0.90), female sex (HR, 0.64; 95% CI, 0.58-0.70), history of cancer (HR, 0.61; 95% CI, 0.47-0.79), history of tobacco consumption (HR, 0.80; 95% CI, 0.73-0.88), high body mass index (≥30: HR, 0.75; 95% CI, 0.63-0.89), and high number of symptoms during the acute phase (>4; HR, 0.43; 95% CI, 0.39-0.48) were associated with a slower resolution of symptoms. In this cross-sectional study, persistent symptoms were still present in 10.1% of infected individuals at 1 year after SARS-CoV-2 infection. Given the high level of cumulative incidence of COVID-19, the absolute prevalent number of people with persistent symptoms is a public health concern.
Situation Dashboards

World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)

Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU

COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources

Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.