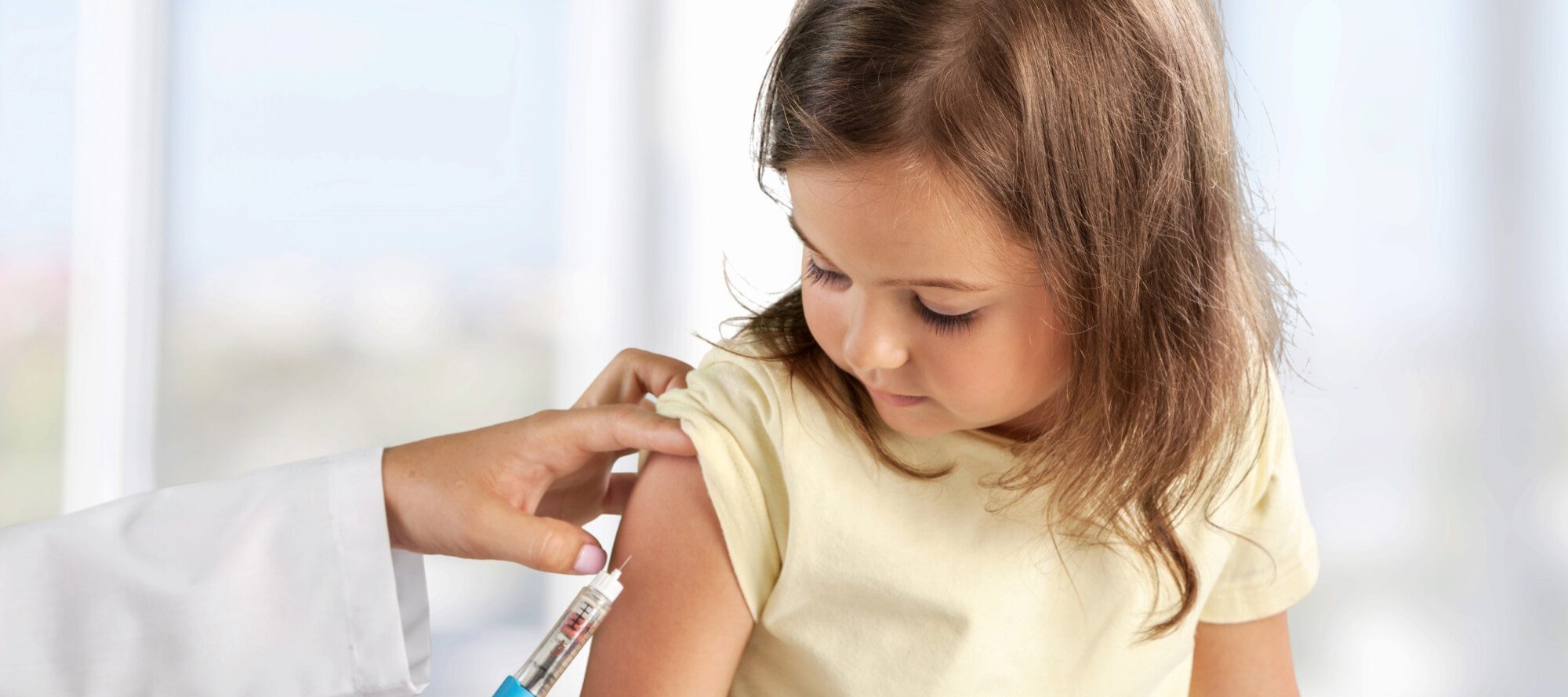
- Durability of Original Monovalent mRNA Vaccine Effectiveness Against COVID-19 Omicron–Associated Hospitalization in Children and Adolescents — United States, 2021–2023
Vaccine effectiveness (VE) of ≥2 original monovalent COVID-19 vaccine doses against COVID-19–related hospitalizations (December 19, 2021–October 29, 2023) across 34 Overcoming COVID-19 Network sites was evaluated using a case-control design. Case-patients were children and adolescents aged 5–18 years who were hospitalized for acute COVID-19 and received a positive SARS-CoV-2 test result. Control patients hospitalized for COVID-19–like illness were matched to case-patients by site, age group, and admission date, but received a negative SARS-CoV-2 test result. Critical COVID-19–related illness was defined as receipt of noninvasive or invasive mechanical ventilation, vasoactive infusions, extracorporeal membrane oxygenation, and illness resulting in death. VE of original monovalent mRNA COVID-19 vaccines against COVID-19–related hospitalization was 52% (95% CI = 33–66) when the most recent vaccine dose was received 7–119 days before hospitalization, 19% (95% CI = 2–32) when it was received 120–364 days before hospitalization, and 31% (95% CI = 18–43) if the last dose was received any time within the previous year. VE against critical COVID-19–related illness was 57% (95% CI = 21–76) when the last dose was 7–119 days before hospitalization, not significant when it was received 120–364 days before hospitalization, and 38% (95% CI = 15–55) when the most recent dose was received at any point within the previous year. During the peak of pediatric COVID-19 hospitalizations (December 19, 2021–March 19, 2022), VE was 55% (95% CI = 38–67) against COVID-19–related hospitalizations when the last dose was received a median of 129 days before hospitalization (IQR = 47–198 days) and 79% (95% CI = 59–89) against critical COVID-19–related illness when the last dose was received a median of 132 days before hospitalization.
- Remdesivir is Associated with Reduced Mortality in Patients Hospitalized for COVID-19 Not Requiring Supplemental Oxygen
Investigators used a large, multicenter U.S. hospital database to look at in-hospital mortality among patients hospitalized for COVID-19 not requiring supplemental oxygen at admission between December 2020 and April 2022 receiving or not receiving remdesivir. Patients receiving remdesivir upon hospital admission were matched 1:1 to those not receiving remdesivir during hospitalization using propensity score matching. Cox proportional hazards models were used to assess 14- and 28-day in-hospital mortality or discharge to hospice. Among the 121,336 eligible patients, 58,188 remdesivir-treated patients were matched to 17,574 unique non-remdesivir patients. Overall, 5.4% of remdesivir-treated and 7.3% of non-remdesivir patients died within 14 days, while 8.0% of remdesivir-treated and 9.8% of non-remdesivir patients died within 28 days. Remdesivir treatment was associated with a statistically significant reduction in in-hospital mortality compared to non-remdesivir treatment (14-day adjusted hazard ratio (aHR): 0.75, 95% confidence interval (CI): 0.68-0.83; 28-day aHR: 0.83, 0.76-0.90). This significant mortality benefit endured across the different VOC periods.
- The Persistence of SARS-CoV-2 in Tissues and its Association with Long COVID Symptoms: A Cross-sectional Cohort Study in China
These are the results of a single-center, cross-sectional cohort study done at China–Japan Friendship Hospital in Beijing, China, following the Omicron wave of COVID-19 in December, 2022. Individuals with mild COVID-19 confirmed by PCR or a lateral flow test scheduled to undergo gastroscopy, surgery, or chemotherapy, or scheduled for treatment in hospital for other reasons, at one month, two months, or four months after infection were enrolled in this study. Residual surgical samples, gastroscopy samples, and blood samples were collected approximately one month (18–33 days), two months (55–84 days), or four months (115–134 days) after infection. Telephone follow-up was done at four months post-infection to assess the association between the persistence of SARS-CoV-2 RNA and long COVID symptoms. Between Jan. 3 and April 28, 2023, 317 tissue samples were collected from 225 patients, including 201 residual surgical specimens, 59 gastroscopy samples, and 57 blood component samples. Viral RNA was detected in 16 (30%) of 53 solid tissue samples collected at 1 month, 38 (27%) of 141 collected at 2 months, and seven (11%) of 66 collected at 4 months. Viral RNA was distributed across ten different types of solid tissues, including liver, kidney, stomach, intestine, brain, blood vessel, lung, breast, skin, and thyroid. Subgenomic RNA was detected in 26 (43%) of 61 solid tissue samples tested for subgenomic RNA that also tested positive for viral RNA. Among 213 patients who completed the telephone questionnaire, 72 (34%) reported at least one Long COVID symptom, with fatigue (21%, 44 of 213) being the most frequent symptom. Detection of viral RNA in recovered patients was significantly associated with the development of Long COVID symptoms (odds ratio 5·17, 95% CI 2·64–10·13, p<0·0001). Patients with higher virus copy numbers had a higher likelihood of developing Long COVID symptoms. In an attempt to investigate the potential mechanisms underlying the association between this persistence of viral remnants and Long COVID symptoms, they did transcriptome sequencing of 11 blood vessels and 24 lung tissues. In lung tissues, they observed downregulated genes involved in the innate and adaptive immune defence against pathogens in the viral persistence group, such as KLRD1, FYB1, VAV2, LILRB4, LILRB5, TICAM1, BTK, CD8A, and CD8B. They also noted a significant downregulation of zinc finger protein-related genes in the persistence of viral remnant group, which play a role in defense against SARS-CoV-2. They interpret these findings as suggesting that dysfunction in host immune defense might contribute to poor virus clearance. In the blood vessel samples with persistence of viral remnants, they identified dysregulation of genes related to the complement and coagulation cascades, such as FGG, VTN, F12, FGB, SERPINA1, C5, C1QB, SERPINE2, SERPINA5, and VSIG4. They also observed dysregulation of genes involved in cholesterol metabolism pathways, such as APOC3, APOA1, APOH, APOA2, LIPG, APOC1, SCARB1, CD36, and PLTP, which is consistent with our previous research findings on the plasma proteome of long COVID. Here they conclude that these findings suggest that persistence of viral remnants might affect host cell functions, which could be another contributing factor to the occurrence of Long COVID symptoms.
Situation Dashboards

World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)

Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU

COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources

Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.