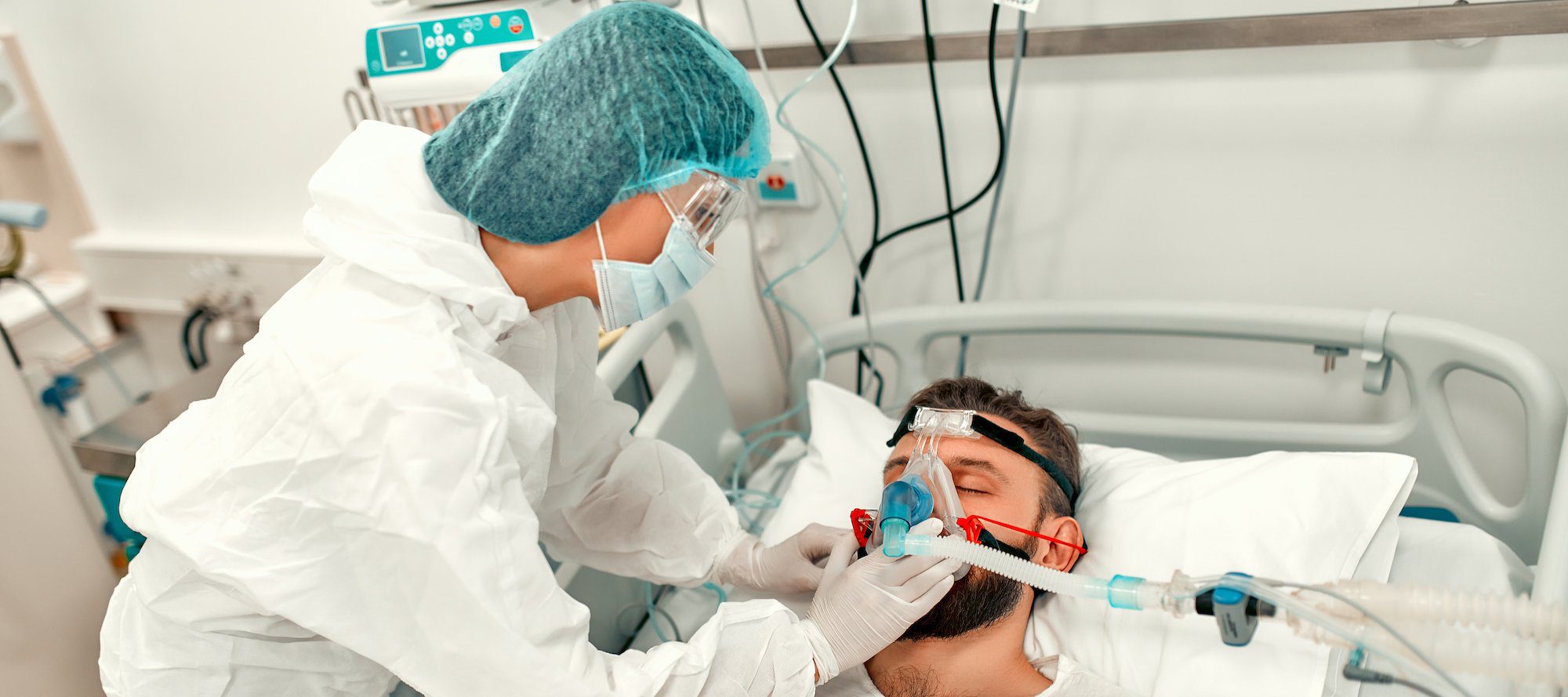
- Outcomes of SARS-CoV-2 Reinfection
First infection with SARS-CoV-2 is associated with increased risk of acute and post-acute death and sequelae in the pulmonary and extrapulmonary organ systems. However, whether reinfection adds to the risk incurred after the first infection is not clear. Here researchers use the national health care databases of the US Department of Veterans Affairs to build a cohort of people with first infection (n = 257,427), reinfection (2 or more infections, n = 38,926), and a non-infected control group (n = 5,396,855) to estimate risks and 6- month burdens of all-cause mortality, hospitalization, and a set of pre-specified incident outcomes. They show that compared to people with first infection, reinfection contributes additional risks of all-cause mortality, hospitalization, and adverse health outcomes in the pulmonary and several extrapulmonary organ systems (cardiovascular disorders, coagulation and hematologic disorders, diabetes, fatigue, gastrointestinal disorders, kidney disorders, mental health disorders, musculoskeletal disorders, and neurologic disorders); the risks were evident in those who were unvaccinated, had 1 shot, or 2 or more shots prior to the second infection; the risks were most pronounced in the acute phase, but persisted in the post-acute phase of reinfection, and most were still evident at 6 months after reinfection. Compared to non-infected controls, assessment of the cumulative risks of repeated infection showed that the risk and burden increased in a graded fashion according to the number of infections. The constellation of findings show that reinfection adds non-trivial risks of all-cause mortality, hospitalization, and adverse health outcomes in the acute and post-acute phase of the reinfection. Reducing overall burden of death and disease due to SARS-CoV-2 will require strategies for reinfection prevention. - Children and COVID-19: State level Data Report
A joint report from the American Academy of Pediatrics and the Children’s Hospital Association. Summary of publicly reported data from 49 states, NYC, DC, PR, and GU Version: 6/16/22. The numbers in this report represent cumulative counts since states began reporting. The data are based on how public agencies collect, categorize and post information. All data reported by state/local health departments are preliminary and subject to change and reporting may change over time. Notably, in the summer of 2021 and winter of 2022, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported. For example, due to several changes on their dashboards and the data currently available, AL, TX, HI, DC and MS data in this report are not current (cumulative data through 7/29/21, 8/26/21, 1/13/22, 3/3/22, and 3/10/22 respectively). Readers should consider these factors. States may have additional information on their web sites.
- CDC Recommends COVID-19 Vaccines for Young Children.
The CDC Director endorsed the Advisory Committee on Immunization Practices’ (ACIP) recommendation that all children 6 months through 5 years of age should receive a COVID-19 vaccine. This expands eligibility for vaccination to nearly 20 million additional children and means that all Americans ages 6 months and older are now eligible for vaccination. - Evaluating clinical effectiveness of SARS-CoV-2 vaccine in solid organ transplant recipients: A propensity score matched analysis
These are the results of a retrospective cohort study comparing SARS-CoV-2 infection rates between SOTR who received 2 doses of mRNA or 1 dose of Ad26.Cov2.S vaccine and those not fully vaccinated (partially vaccinated and unvaccinated). Of 2,705 SOTR, 1668 were included in the final matched analysis, which showed a 73% reduction of SARS-CoV-2 infection and 76% reduction of all-cause-mortality among fully vaccinated patients. Thirty-nine SOTR developed SARS-CoV-2 infection, including 9 fully vaccinated and 30 not fully vaccinated. Among fully vaccinated patients, 22% had severe/critical COVID-19 and 0% mortality versus not fully vaccinated SOTR, of whom 37% had severe/critical COVID-19 and 7% COVID-19-related mortality. All COVID-19 related deaths in this study were in unvaccinated patients Some hospitalizations may have been prevented with use of monoclonal antibodies in the outpatient setting (three patients in the fully vaccinated and nine patients in the not fully vaccinated groups received monoclonal antibody and were not subsequently hospitalized). With regard to measuring the humoral immune response to COVID-19 vaccination and infection, validated cutoffs for many of the wide array of available antibody assays still being investigated –making it difficult to interpret the meaning of a given antibody test. Our results, which show protection from infection, severe/critical disease, hospitalization, and death among fully vaccinated SOTR, suggest that measurement of antibodies should not be the sole surrogate marker for vaccine effectiveness. - Therapeutic efficacy of monoclonal antibodies and antivirals against SARS-CoV-2 Omicron BA.1 in Syrian hamsters
The spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the major antigen stimulating the host’s protective immune response. Here we assessed the efficacy of therapeutic monoclonal antibodies (mAbs) against Omicron variant (B.1.1.529) sublineage BA.1 variants in Syrian hamsters. Of the FDA-approved therapeutic mAbs tested (that is, REGN10987/REGN10933, COV2-2196/COV2-2130 and S309), only COV2-2196/COV2-2130 efficiently inhibited BA.1 replication in the lungs of hamsters, and this effect was diminished against a BA.1.1 variant possessing the S-R346K substitution. In addition, treatment of BA.1-infected hamsters with molnupiravir (a SARS-CoV-2 RNA-dependent RNA polymerase inhibitor) or S-217622 (a SARS-CoV-2 protease inhibitor) strongly reduced virus replication in the lungs. These findings suggest that the use of therapeutic mAbs in Omicron-infected patients should be carefully considered due to mutations that affect efficacy, and demonstrate that the antiviral compounds molnupiravir and S-217622 are effective against Omicron BA.1 variants. - Rebound Phenomenon after Nirmatrelvir/Ritonavir Treatment of Coronavirus Disease-2019 in High-Risk Persons
In a cohort of 483 high-risk patients treated with nirmatrelvir/ritonavir for coronavirus disease-2019, two patients (0.4%) required hospitalization by day 30. Four patients (0.8%) experienced rebound of symptoms, which were generally mild, at median of 9 days after treatment, and all resolved without additional COVID-19-directed therapy. - Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment — California, December 2021–May 2022
This study found that <1% of patients treated with Paxlovid were identified with COVID-19–related hospitalization or ED encounters 5-15 days after treatment was dispensed. When administered as an early-stage treatment, Paxlovid might prevent COVID-19–related hospitalization among persons with mild to moderate COVID-19 cases who are at risk for progression to severe disease. Additional research is warranted to provide further understanding of the apparent association between Paxlovid and reduced risk for severe COVID-19 illness, including studies with control groups and more precise indicators of COVID-19 illness severity.
- Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy
These are the results of a cohort study of 7772 infants delivered during the COVID-19 pandemic. In this cohort 222 mothers had a positive SARS-CoV-2 polymerase chain reaction test during pregnancy. Maternal SARS-CoV-2 positivity during pregnancy was associated with greater rate of neurodevelopmental diagnoses in unadjusted models (odds ratio [OR], 2.17 [95% CI, 1.24-3.79];P = .006). - Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2
In this study, researchers found a reduction in odds of long COVID with the omicron variant versus the delta variant of 0·24–0·50 depending on age and time since vaccination. However, the absolute number of people experiencing long COVID at a given time depends on the shape and amplitude of the pandemic curve. For example, given the high numbers of people infected with omicron in the UK from December, 2021, to February, 2022, our data are consistent with the UK Office for National Statistics, who estimated that the numbers of people experiencing long COVID actually increased from 1·3 million in January, 2022, to 1·7 million in March, 2022. Considering the UK omicron peak of more than 350 000 new symptomatic COVID-19 cases per day estimated on March 26, 2022, by the ZOE app model and 4% of cases being long COVID, future numbers with long COVID will inevitably rise. - Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross- sectional study
A nationwide cross-sectional study was conducted including children with a confirmed SARS-CoV-2-positive PCR test (cases) and matched controls from Danish national registers. A survey was sent to mothers (proxy reporting) of children aged 0–14 years who had had a positive SARS-CoV-2 test between Jan 1, 2020, and July 12, 2021, and a control group matched (1:4) by age and sex. The survey included the Pediatric Quality of Life Inventory (PedsQL) and the Children's Somatic Symptoms Inventory-24 (CSSI-24) to capture current overall health and wellbeing, and ancillary questions about the 23 most common long COVID symptoms. Descriptive statistics and logistic regression analysis were used. Clinically relevant differences were defined as those with a Hedges' gscore greater than 0·2. Responses to the survey were received from 10 997 (28·8%) of 38 152 cases and 33 016 (22·4%) of 147 212 controls between July 20, 2021, and Sept 15, 2021. Median age was 10·2 years (IQR 6·6–12·8) in cases and 10·6 years (6·9–12·9) in controls. 5267 (48·2%) cases and 15 777 (48·3%) controls were female, and 5658 (51·8%) cases and 16 870 (51·7%) controls were male. Cases had higher odds of reporting at least one symptom lasting more than 2 months than did controls in the 0–3 years age group (478 [40·0%] of 1194 vs 1049 [27·2%] of 3855; OR 1·78 [95% CI 1·55–2·04], p<0·0001), 4–11 years age group (1912 [38·1%] of 5023 vs 6189 [33·7%] of 18 372; 1·23 [1·15–1·31], p<0·0001), and 12–14 years age group (1313 [46·0%] of 2857 vs 4454 [41·3%] of 10 789; 1·21 [1·11–1·32], p<0·0001). Differences in CSSI-24 symptom scores between cases and controls were statistically significant but not clinically relevant. Small clinically relevant differences in PedsQL quality-of-life scores related to emotional functioning were found in favor of cases in the children aged 4–11 years (median score 80·0 [IQR 65·0–95·0]) in cases vs 75·0 [60·0–85·0] in controls; p<0·0001) and 12–14 years (90·0 [70·0–100·0] vs (85·0 [65·0–95·0], p<0·0001). PedsQL social functioning scores were also higher in cases (100·0 [90·0–100·0] than controls (95·0 [80·0–100·0]) in the 12–14 years age group (p<0·0001; Hedges g>0·2). Compared with controls, children aged 0–14 years who had a SARS-CoV-2 infection had more prevalent long-lasting symptoms. There was a tendency towards better quality-of-life scores related to emotional and social functioning in cases than in controls in older children. The burden of symptoms among children in the control group requires attention. Long COVID must be recognized and multi-disciplinary long COVID clinics for children might be beneficial.
Situation Dashboards



World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)


Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU


COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources


Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.