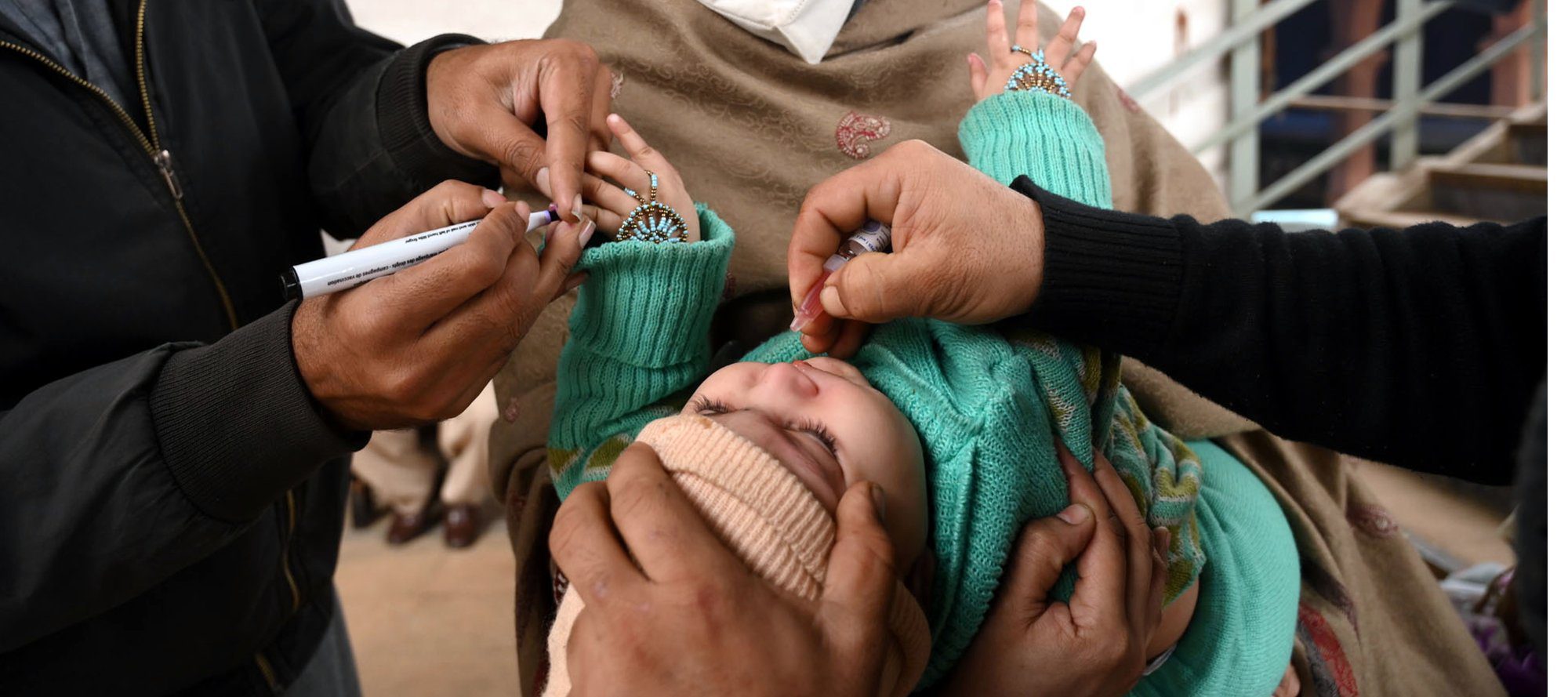
- Progress Toward Poliomyelitis Eradication — Pakistan, January 2021–July 2022
Pakistan is one of two countries (including Afghanistan) where wild poliovirus type 1 (WPV1) transmission has never been interrupted. WPV1 cases in Pakistan decreased from 147 in 2019 and 84 in 2020 to a single case in 2021 but increased to 14 cases in 2022 as of July 31. These 14 WPV1 cases are clustered among children in southern Khyber Pakhtunkhwa province, many of whom have never received poliovirus vaccine (zero-dose children). Ensuring the highest quality vaccination activities in priority areas of Pakistan will enable the polio program to improve the chances of interrupting ongoing transmission of WPV1. - Polio by the Numbers—A Global Perspective
Investments in national immunization programs and the Global Polio Eradication Initiative (GPEI) have resulted in substantial reductions in paralytic polio worldwide. However, cases prevented because of investments in immunization programs and GPEI remain incompletely characterized. Using a global model that integrates polio transmission, immunity, and vaccine dynamics, researchers provide estimates of polio incidence and numbers of paralytic cases prevented. They compare the results with reported cases and estimates historically published by the World Health Organization. Researchers estimate that the existence and use of polio vaccines prevented 5 million cases of paralytic polio for 1960–1987 and 24 million cases worldwide for 1988–2021 compared to a counterfactual world with no polio vaccines. Since the 1988 resolution to eradicate polio, their estimates suggest GPEI prevented 2.5–6 million cases of paralytic polio compared to counterfactual worlds without GPEI that assume different levels of intensity of polio vaccine use in routine immunization programs. Analysis of historical cases provides important context for understanding and communicating the benefits of investments made in polio eradication. Prospective studies will need to explore the expected benefits of future investments, the outcomes of which will depend on whether and when polio is globally eradicated.
- Phase 1/2a Safety and Immunogenicity of an Adenovirus 26 Vector RSV Vaccine Encoding Prefusion F in Adults 18–50 Years and RSV Seropositive Children 12–24 Months
Respiratory syncytial virus (RSV) remains a leading cause of pediatric morbidity, with no approved vaccine. Researchers assessed the safety and immunogenicity of the Ad26.RSV.preF vaccine candidate in adults and children. In this randomized, double-blind, phase 1/2a, placebo-controlled study, 12 adults (18–50 years) and 36 RSV-seropositive children (12–24 months) were randomized 2:1 to Ad26.RSV.preF (1 × 1011viral particles [vp] for adults, 5 × 1010 vp for children) or placebo, at day 1 and 29, with 6-month immunogenicity and 1-year safety follow-up. RSV infection was an exploratory outcome in children. In adults, solicited adverse events (AEs) were generally mild–moderate, with no serious AEs. In children, no vaccination-related serious AEs were reported; fever was reported in 14 (58.3%) Ad26.RSV.preF recipients. Baseline pediatric geometric mean titers for RSV A2 neutralization increased from 121 (95% confidence interval [CI], 76–191) to 1608 (95% CI, 730–3544) at day 29, and 2235 (95% CI, 1586–3150) at day 57, remaining elevated over 7 months. RSV infection was confirmed in fewer children receiving Ad26.RSV.preF (1, 4.2%) than placebo (5, 41.7%). Ad26.RSV.preF demonstrated immunogenicity in healthy adults and toddlers, with no safety concerns raised. Evaluations in RSV-seronegative children are underway. - Receipt of First and Second Doses of JYNNEOS Vaccine for Prevention of Monkeypox — United States, May 22–October 10, 2022
In the United States, JYNNEOS vaccine is recommended for persons exposed to or at high risk for exposure to Monkeypox virus. By October 10, 2022, a total of 931,155 JYNNEOS vaccine doses were administered in the United States. Among persons who received ≥1 vaccine dose, 51.4% were non-Hispanic White, 12.6% were non-Hispanic Black or African American (Black), and 22.5% were Hispanic persons. The percentages of vaccine recipients who were Black (5.6%) and Hispanic (15.5%) during May 22–June 25 increased to 13.3% and 22.7%, respectively, during July 31–October 10. Tracking and addressing disparities in vaccination can reduce inequities and help ensure that disproportionately affected populations are protected. - Novavax NVX-COV2373 triggers potent neutralization of Omicron sub-lineages
The SARS-CoV-2 Omicron (B.1.1.529) Variant of Concern (VOC) and its sub-lineages (including BA.2, BA.4/5, BA.2.12.1) contain spike mutations that confer high level resistance to neutralizing antibodies. The NVX-CoV2373 vaccine, a protein nanoparticle vaccine, has value in countries with constrained cold-chain requirements. Here researchers report neutralizing titers following two or three doses of NVX-CoV2373. Researchers show that after two doses, Omicron sub-lineages BA.1 and BA.4 were resistant to neutralization by 72% (21/29) and 59% (17/29) of samples. However, after a third dose of NVX-CoV2373, they observed high titers against Omicron BA.1 (GMT: 1,197) and BA.4 (GMT: 582), with responses similar in magnitude to those triggered by three doses of an mRNA vaccine. These data are of particular relevance as BA.4 is emerging to become the dominant strain in many locations, and highlight the potential utility of the NVX-CoV2373 vaccine as a booster in resource-limited environments. - COVID-19 Outcomes in Solid Organ Transplant Recipients Who Received Tixagevimab-cilgavimab Prophylaxis and/or Bebtelovimab Treatment in a Nurse-driven Monoclonal Antibody Program During the Omicron Surge
Despite large numbers of cases during the Omicron surge, SOTRs who received T/C were unlikely to contract COVID-19 and rarely required hospitalization or died. Several cardiac events were reported, but the relationship to T/C is unclear. Of those who received BEB for treatment, few required hospitalization, and only 1 (0.7%) died. These favorable outcomes underscore the importance of a systematized, nurse-led program for monoclonal antibody prophylaxis or treatment in SOTRs. - Omicron sublineage BA.2.75.2 exhibits extensive escape from neutralizing antibodies
Several sublineages of omicron have emerged with additional mutations that may afford further antibody evasion. Here, researchers characterize the sensitivity of emerging omicron sublineages BA.2.75.2, BA.4.6, and BA.2.10.4 to antibody-mediated neutralization, and identify extensive escape by BA.2.75.2. BA.2.75.2 was resistant to neutralization by Evusheld (tixagevimab + cilgavimab), but remained sensitive to bebtelovimab. In recent serum samples from blood donors in Stockholm, Sweden, BA.2.75.2 was neutralized, on average, five-fold less potently than BA.5, representing the most neutralization resistant variant evaluated to date. These data raise concerns that BA.2.75.2 may effectively evade humoral immunity in the population.
- Influenza and COVID-19 Vaccination Coverage Among Health Care Personnel
Influenza and COVID-19 vaccines are recommended for all persons aged ≥6 months, including health care personnel (HCP). HCP influenza vaccination coverage was 79.9% during the 2021–22 season; 87.3% completed primary COVID-19 vaccination, 67.1% of whom received a COVID-19 booster dose. Influenza, primary COVID-19, and COVID-19 booster coverage was higher among HCP who reported employer vaccination requirements for those vaccines; coverage was lowest among HCP working in long-term care settings. Enhanced efforts are needed to improve HCP vaccination coverage, especially with COVID-19 booster doses and annually for influenza vaccines. Staying up to date with COVID-19 and influenza vaccines can protect HCP and their patients. - Distinguishing SARS-CoV-2 persistence and reinfection
SARS-CoV-2 reinfection is poorly understood, partly because few studies have systematically applied genomic analysis to distinguish reinfection from persistent RNA detection related to initial infection. Researchers aimed to evaluate the characteristics of SARS-CoV-2 reinfection and persistent RNA detection using independent genomic, clinical, and laboratory assessments. All individuals at a large academic medical center who underwent a SARS-CoV-2 nucleic acid amplification test (NAAT) ≥ 45 days after an initial positive test, with both tests between March 14thand December 30th, 2020, were analyzed for potential reinfection. Inclusion criteria required having ≥2 positive NAATs collected ≥45 days apart with a cycle threshold (Ct) value <35 at repeat testing. For each included subject, likelihood of reinfection was assessed by viral genomic analysis of all available specimens with a Ct value <35, structured Ct trajectory criteria, and case-by-case review by infectious diseases physicians. Among 1,569 individuals with repeat SARS-CoV-2 testing ≥45 days after an initial positive NAAT, 65 (4%) met cohort inclusion criteria. Viral genomic analysis characterized mutations present, and was successful for 14/65 (22%) subjects. Six subjects had genomically-supported reinfection and eight subjects had genomically-supported persistent RNA detection. Compared to viral genomic analysis, clinical and laboratory assessments correctly distinguished reinfection from persistent RNA detection in 12/14 (86%) subjects but missed 2/6 (33%) genomically-supported reinfections. Despite good overall concordance with viral genomic analysis, clinical and Ct value-based assessments failed to identify 33% of genomically-supported reinfections. Scaling-up genomic analysis for clinical use would improve detection of SARS-CoV-2 reinfections. - Association between regular physical activity and the protective effect of vaccination against SARS-CoV-2 in a South African case–control study
Both vaccination and physical activity have been shown to independently decrease the likelihood of severe COVID-19 infection. A test negative case–control study design was used to estimate the risk of having an associated COVID-19-related hospital admission, among individuals who were unvaccinated compared with those who were fully vaccinated with Ad26.COV2.S (>28 days after a single dose). 196,444 participant tests were stratified into three measured physical activity subgroups with low, moderate and high activity, to test the hypothesis that physical activity is an effect modifier on the relationship between vaccination and hospitalization. Vaccine effectiveness against a COVID-19-related admission among vaccinated individuals within the low activity group was 60.0% (95% CI 39.0 to 73.8), 72.1% (95% CI 55.2 to 82.6) for the moderate activity group, and 85.8% (95% CI 74.1 to 92.2) for the high activity group. Compared with individuals with low activity levels, vaccinated individuals with moderate and high activity levels had a 1.4 (95% CI 1.36 to 1.51) and 2.8 (95% CI 2.35 to 3.35) times lower risk of COVID-19 admission, respectively (p value <0.001 for both groups). Regular physical activity was associated with improved vaccine effectiveness against COVID-19 hospitalization, with higher levels of physical activity associated with greater vaccine effectiveness. Physical activity enhances vaccine effectiveness against severe COVID-19 outcomes and should be encouraged by greater public health messaging. - Tocilizumab vs. Baricitinib in hospitalized patients with severe COVID-19
Researchers assigned 251 patients with COVID-19 and PaO2/FiO2<200 to receive either tocilizumab (n=126) or baricitinib (n=125) plus standard of care. Baricitinib was non-inferior to tocilizumab for the primary composite outcome of mechanical ventilation or death by day 28 [mechanical ventilation or death-baricitinib: 39.2% (n=49/125), tocilizumab: 44.4% (n=56/126), HR 0.83, 95% CI: 0.56 to 1.21, p=0.001 for non-inferiority]. Baricitinib was non-inferior to tocilizumab for the time to hospital discharge within 28 days (discharged alive-baricitinib: 58.4% (n=73/125) vs tocilizumab: 52.4% (n=66/126), HR 0.85, (95% CI: 0.61 to 1.18), p<0.001 for non-inferiority). There was no significant difference between baricitinib and tocilizumab arm in the change in WHO scale at day 10 [0.0 (95% CI: 0.0 to 0.0) vs 0.0 (95% CI: 0.0 to 1.0), p=0.83]. In the setting of this trial, baricitinib was non-inferior to tocilizumab with regards to the composite outcome of mechanical ventilation or death by day 28 and the time to discharge by day 28 in patients with severe COVID-19.
Situation Dashboards



World Health Organization (WHO)
Novel Coronavirus (COVID-19) Situation from World Health Organization (WHO)


Johns Hopkins University (JHU)
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at JHU


COVID-19 in US and Canada
1Point3Acres Real-Time Coronavirus (COVID-19) Updates in US and Canada with Credible Sources


Genomic Epidemiology COVID-19
Genomic Epidemiology of (COVID-19) Maintained by the Nextstrain team, enabled by data from GISAID.